
117
Pickling and passivation
A stainless steel surface will have excellent corrosion resistance
due to the chromium oxide layer on the surface of the product.
With some stainless steels however, the surface areas can
become subject to corrosion due to the depletion of chromium
during welding, or the introduction of iron during a machining
process (not applicable to most cable management products).
Where a uniform appearance is important after carrying out
welding processes, it is often specified that all surfaces should
be pickled and passivated to remove the smoke stain from the
welding process. Also where extreme corrosion resistance is
called for, this process may help to remove crevice corrosion from
around the welding area.
Pickling
The pickling process involves the article being immersed in a
blend of acids which dissolve iron and iron oxides which adhere to,
or are embedded in, the surface of the stainless steel. These acids
cause a removal of the surface layer of between 1 and 3 microns.
The article is finally rinsed with water to complete this stage of
the process.
Passivation
Passivation of the stainless steel will occur naturally after pickling
when the oxygen in the air will react with the surface of the steel
to form a passive chromium oxide layer. However it is usual for
this passivation process to be speeded up by immersing the article
in a nitric acid or other passivating agent.
Pickling and passivation gives Cablofil stainless steel wire cable
tray a very light grey colour and a distinctly matt finish.
All Cablofil stainless steel products are pickled and passivated.
2
Stainless steels
For all practical purposes most stainless steel services supports
can be regarded as maintenance free and suffering no corrosion.
Inevitably there is a relatively high price to pay for these attractive
properties but, in aggressive environments or where the cost
or inconvenience of gaining subsequent maintenance access is
prohibitive, this initial cost premium may well be justified.
Background
Stainless steel contains a high proportion of chromium (usually
at least 17%) and the steel’s remarkable immunity to corrosive
attack is conferred by the chromium-rich oxide film which occurs
naturally on its surface. This invisible film is not only inert and
tightly bonded to the surface, it also re-forms quickly if the
surface is damaged in any way.
The fire resistance of stainless steel is particularly noteworthy;
tests have demonstrated that stainless steel cable supports can
be expected to maintain their integrity for considerable periods
even when exposed to direct flame temperatures exceeding
1,000°C. This may be an important consideration where the
electrical circuits being supported provide for emergency power
or control systems.
Stainless steel is also used where hygiene is a major
consideration. Its advantages in such applications are again its
excellent resistance to the various chemicals and washes which
are frequently used for cleaning purposes and the smoothness of
surface (depending on the finish specified) which minimises the
soiling or contamination that can take place.
Stainless steel 304 L
EN 10088-2 standard AISI 304L – X2CrNi18.09 – 1.4307
Offers good corrosion resistance against soft water, normal
environments and food products (except mustard and white wine).
Stainless steel 316 L
EN 10088-2 standard AISI 316L – X2CrNiMo17.12.2 – 1.4404
Since it contains molybdenum, stainless steel 316L is able to
resist intergranular corrosion. This makes it particularly suitable
for the chemical and food industries, the nitrate explosives
industry and environments containing halogen (fluorine and
chlorine).
100
200
400
600
800
1000
1200
1400
1500
Pickled
& passivated
Pickled
& passivated
316L
Un-
treated
Un-
treated
Stainless
steel
Carbon
steel
304L
316L
EZ
GS
GC
304L
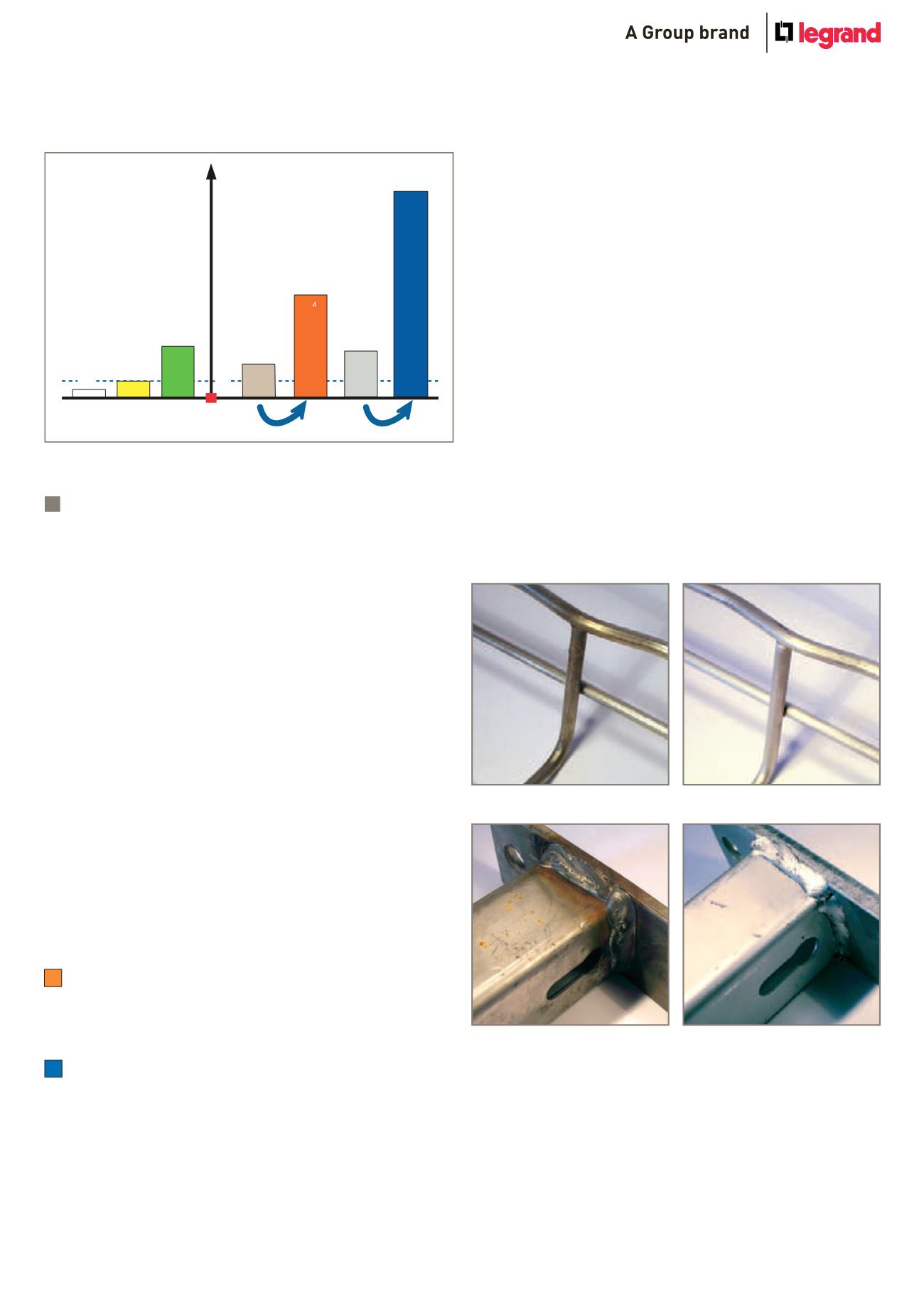
x 4
x 5
300
Figures for salt spray tests, baseline 100 hours : EZ
316L
304L
Pickled and passivated
Untreated
Pickled and passivated
Untreated



















