
Lighting Technology
Fundamentals of Light and Colour
Radiation
Radiation is the emission and transmission of energy in
the form of electro-magnetic waves of a speciic frequency
and wavelength. The propagation velocity of the electro-
magnetic waves in vacuum is approx. 300,000 km/s
independent of the frequency. In radioparent gases, luids
and solid bodies the propagation velocity is always less
than in vacuum. Most physical phenomena related to the
propagation of radiation can be explained with the theory
of electro-magnetic waves.
Interactions between matter and radiation though are
explained with quantum theory. It states that energy is
emitted and absorbed only in elementary quantities by
so called quantums or photons. Examples of phenomena
taken from quantum theory are photo electric, chemical or
biological efects.
2. Optical radiation
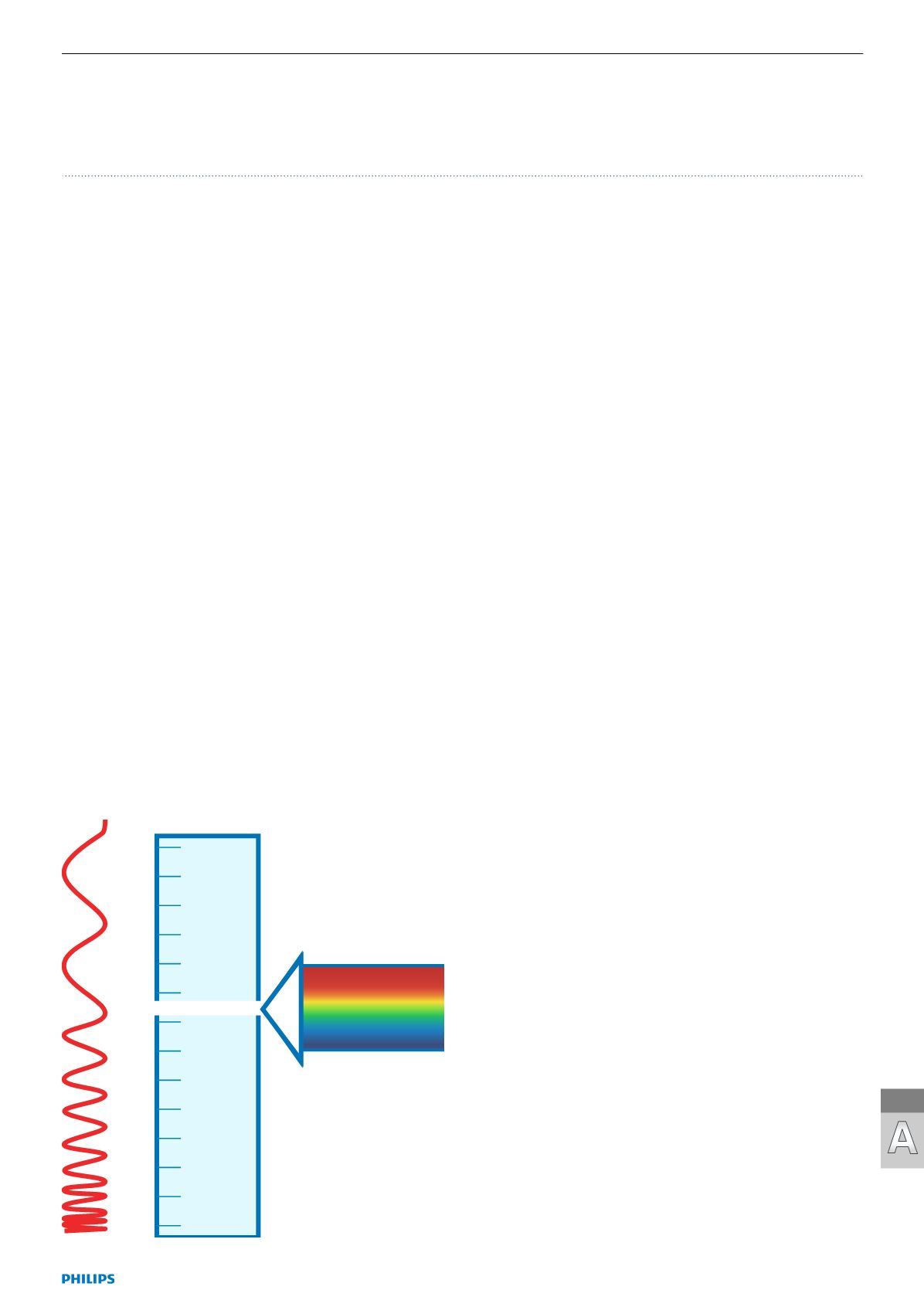
Optical radiation represents a small part of the spectrum of
electromagnetic waves of about 1 nm to 1 mm wavelength. It
comprises visible radiation, which stimulates the sensation
of brightness in the eye and is called light as well as the two
adjacent kinds of radiation in the spectrum.
These two are ultra-violet radiation (UV) in the direction
of shorter wavelengths and infrared radiation (IR) in the
direction of longer wavelengths. The behaviour of the
various kinds of optical radiation is largely similar, they can
all be generated by artiicial radiation sources and directed
using optical modules.
Optical radiation is subdivided as follows, taking into
account the fact that the boundaries cannot be clearly
deined and that, in the case of light, they depend on the
individual person’s visual faculties:
Ultra-violet radiation:
Infrared radiation:
UV-C 100 nm – 280 nm
IR-A 780 nm – 1,4 μm
UV-B 280 nm – 315 nm
IR-B 1,4 μm – 3,0 μm
UV-A 315 nm – 380 nm
IR-C 3,0 μm – 1,0 mm
Visible radiation:
Light 380 nm – 780 nm
UV-C radiation has a bactericide efect and causes erythem
(redness of the skin) and conjunctivitis. Radiation with
wavelengths under 200 nm generates ozone from oxygen.
UV-B radiation causes erythem and generates vitamin D in
the body. UV-A radiation tans the skin without causing a
sunburn. It stimulates certain substances to generate
luorescence and is therefore used to analyze cheques
and bank notes as well as to create decorative efects
in advertising. Infrared radiation (IR) is being absorbed
by material and transformed into heat especially in the
shortwave range.
The spectrum of visible radiation (light) can be subdivided
into various wavelength ranges, causing certain colour
sensations in the human eye:
violet 380 nm – 436 nm yellow 566 nm – 589 nm
blue 436 nm – 495 nm
orange 589 nm – 627 nm
green 495 nm – 566 nm red
627 nm – 780 nm
3. Spectral luminosity of the eye
The human eye exhibits various sensitivities to visible
radiation depending on its wavelength. Although
possessing the same eiciency, a light stimulus of 555
nm e.g. is perceived as much brighter than light stimuli
of 400 nm (violet) or 700 nm (red). The CIE has deined
the spectral luminosity V (
λ
) for the normal sighted eye in
daylight and V’ (
λ
) for sight during the night depending on
the respective wavelength.
Wavelength and Radiation
LW
MW
KW
UKW
TV
Radar
UV
X-ray
Gamma
Space
radiation
nm
10
13
10
11
10
9
10
7
10
5
10
3
10
10
-1
10
-3
10
-5
10
-7
10
-9
10
-11
10
-13
IR
Visible Light
Appendix>
Lighting Technology
Philips Lamps and Lighting Electronic Catalogue 2014
235
13


















