
115
3
The merits of Zinc
The Galvanic Series does show why zinc is such a useful corrosion
resistant coating for mild steel.
Firstly, it forms an impervious zinc barrier around the steel,
coating it with a metal whose own rate of chemical corrosion is
both low and predictable in most situations.
Secondly, if the coating is damaged at any point (e.g. at a cut edge)
the zinc surrounding the damaged area becomes the anode of the
electrolytic cell and is sacrificially corroded away very slowly in
preference to the underlying steel. This ensures the strength of
the steel structure remains unaffected.
Because zinc appears near the top of the Galvanic Series it will
act as a sacrificial anode in relation to most other metals; thus its
relatively low cost and the ease with which it can be applied as a
galvanised coating on steel means that it continues to be the most
commonly specified protective finish for support systems.
Life expectancy of zinc coatings
The resistance of galvanising to atmospheric corrosion depends
on a protective film which forms on the surface of the zinc. When
the steel is withdrawn from the galvanising bath the zinc has
a clean, bright, shiny surface. Over time the appearance will
change to a dull grey patina as the surface reacts with oxygen,
water and carbon dioxide in the atmosphere. A complex but
tough, stable and protective layer is formed which adheres to the
zinc. Contaminants in the atmosphere affect the nature of this
protective film.
The most significant contaminant which will accelerate the
corrosion rate of zinc is sulphur dioxide (S02) and it is the
presence of S02 which largely controls the atmospheric corrosion
of zinc.
The Zinc Millennium Map
The Galvanizers Association has undertaken significant research
based upon the positioning of reference canisters placed
throughout the UK and the Republic of Ireland to establish
background corrosion rates for 10 km
2
grids which has resulted in
the formation of The Zinc Millennium Map.
With the correct use of the map specific locations can be analysed
for average zinc corrosion rates per year.
Further information is available at
www.galvanizing.org.uk.
4
Common corrosion situations
The most common occurrences of contact between dissimilar
metals within support systems are :
a. Where stainless steel components are being fixed to a carbon
steel structure
b. Where galvanised or zinc plated components are being fixed
onto a stainless steel support system
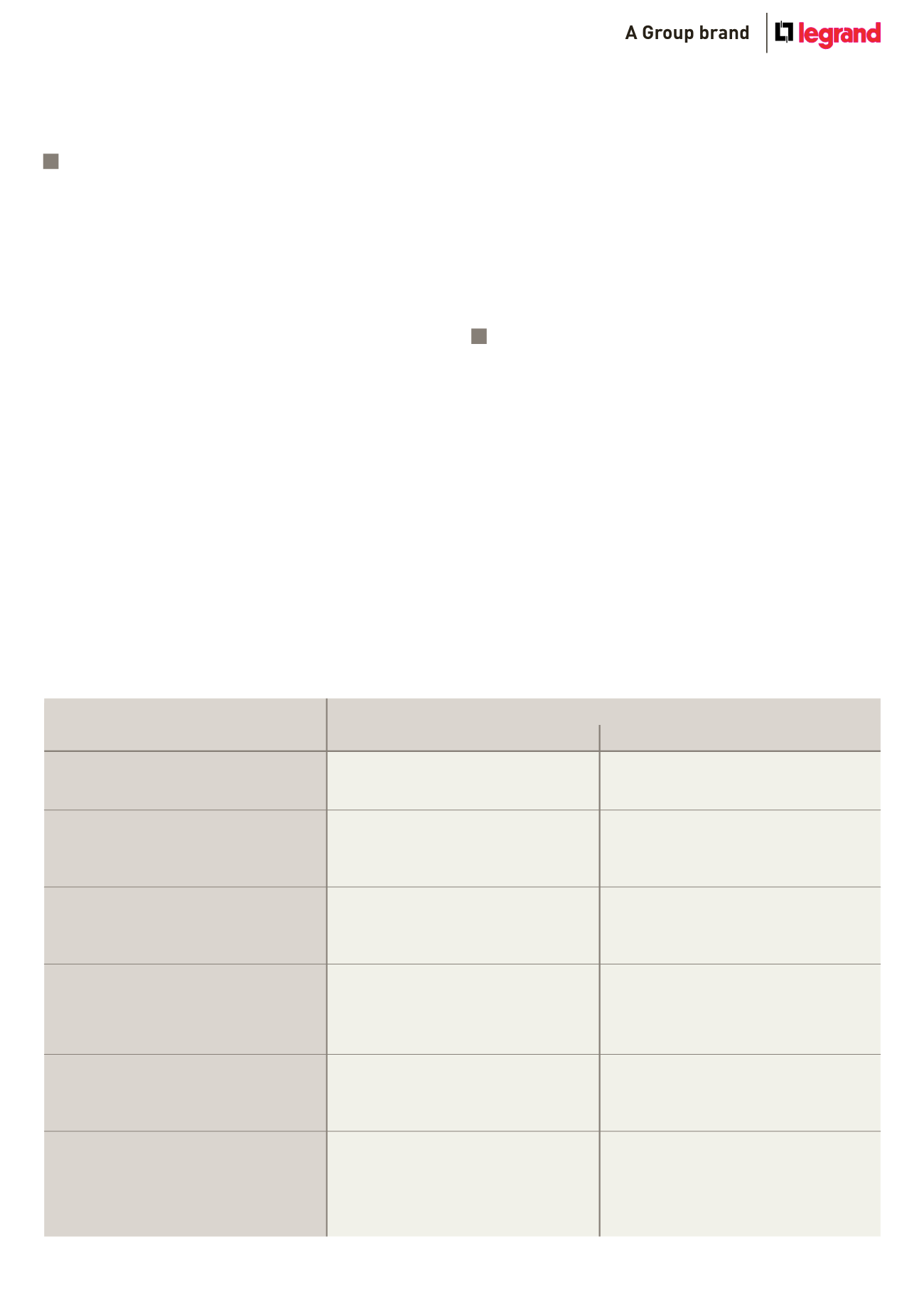
Description of typical atmospheric enviroments related to the estimation of corrosivity categories
Corrosivity category C. Corrosion rate for
zinc (based upon one year exposures),
rcorr (μm.a-1) and corrosion level
Indoor
Outdoor
Typical enviroments (examples)
C1
rcorr ² 0.1
Very low
Heated spaces with low relative humidity and
insignificant pollution, e.g. offices, schools, museums
Dry or cold zone, atmospheric environment with very
low pollution and time of wetness, e.g. certain deserts,
central Arctic / Antarctica
C2
0.1 < rcorr ² 0.7
Low
Unheated spaces with varying temperature and
relative humidity. Low frequency of condensation and
low pollution, e.g. storage, sport halls
Temperate zone, atmospheric environment with low
pollution (SO2 < 5 μg/m3), e.g.: rural areas, small
towns. Dry or cold zone, atmospheric environment
with short time of wetness, e.g. deserts, sub-arctic
areas
C3
0.7 < rcorr ² 2
Medium
Spaces with moderate frequency of condensation
and moderate pollution from production process, e.g.
foodprocessing plants, laundries, breweries, dairies
Temperate zone, atmospheric environment with
medium pollution (SO2: 5 μg/m3 to 30 μg/m3) or some
effect of chlorides, e.g. urban areas, coastal areas with
low deposition of chlorides, subtropical and tropical
zones with atmosphere with low pollution
C4
2 < rcorr ² 4
High
Spaces with high frequency of condensation and high
pollution from production process, e.g. industrial
processing plants, swimming pools
Temperate zone, atmospheric environment with high
pollution (SO2: 30 μg/m3 to 90 μg/m3) or substantial
effect of chlorides, e.g. polluted urban areas, industrial
areas, coastal areas without spray of salt water, exposure
to strong effect of de-icing salts, subtropical and tropical
zones with atmosphere with medium pollution
C5
4 < rcorr ² 8
Very high
Spaces with very high frequency of condensation and/
or with high pollution from production process, e.g.
mines, caverns for industrial purposes, unventilated
sheds in subtropical and tropical zones
Temperate and subtropical zones, atmospheric
environment with very high pollution (SO2: 90 μg/m3 to
250 μg/m3) and/or important effect of chlorides,
e.g. industrial areas, coastal areas, sheltered positions
on coastline
CX
8 < rcorr ² 25
Extreme
Spaces with almost permanent condensation or
extensive periods of exposure to extreme humidity
effects and/or with high pollution from production
process, e.g. unventilated sheds in humid tropical
zones with penetration of outdoor pollution including
airborne chlorides and corrosion-stimulating
particulate matter
Subtropical and tropical zones (very high time of
wetness), atmospheric environment with very high
pollution (SO2 higher than 250 μg/m3), including
accompanying and production pollution and/or strong
effect of chlorides, e.g. extreme industrial areas,
coastal and offshore areas with occasional contact
with salt spray



















